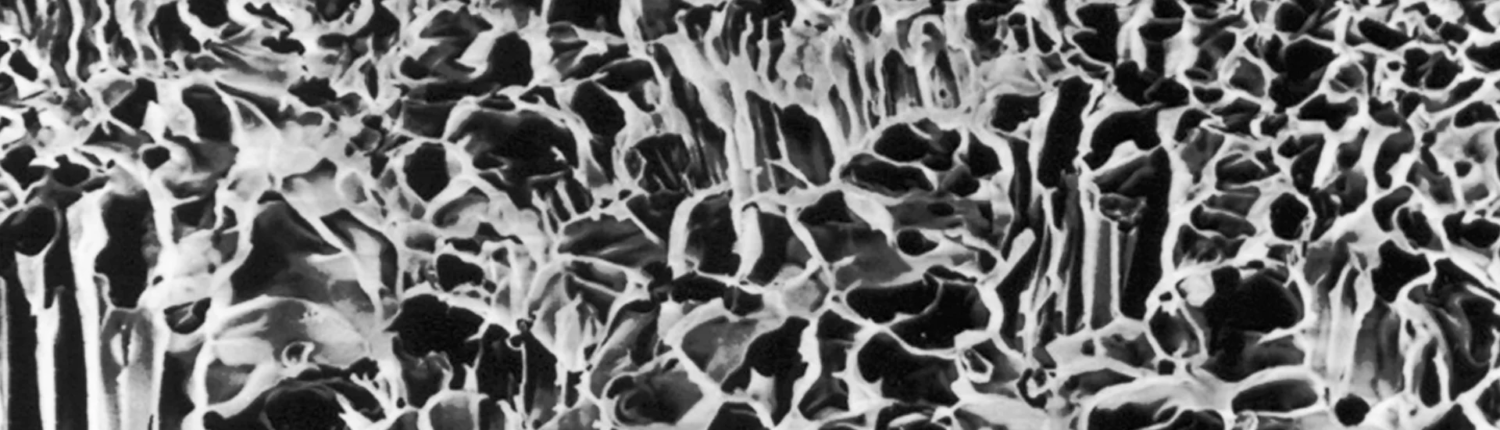
Resorbable membrane
EPI-GUIDE®
Resorbable polylactic acid (PLA) membrane
EPI-GUIDE® is a non-biologic, resorbable, hydrophilic membrane containing a three-dimensional structure important for barrier function. Its three-dimensional constructed density gradient is designed to attract and stabilize fibroblasts and epithelial cells while allowing permeable nutrients through the membrane.
Benefits
- Non-biologic and resorbable
- Resists migration with excellent adherence
- Extended barrier function
- High absorption capacity
- Textured surface
- Benefits open healing